CTPH provides consortia members access to a multitude of services that support clinical and translational research.
Study Design, Data & Analysis

Study design, data and analysis services are offered by Baylor College of Medicine’s Institute for Clinical and Translational Research (ICTR), and the University of Houston’s Texas Institute for Measurement, Evaluation, and Statistics (TIMES) and Hewlett Packard Enterprise Data Science Institute (HPEDSI).
Clinical Trials Support
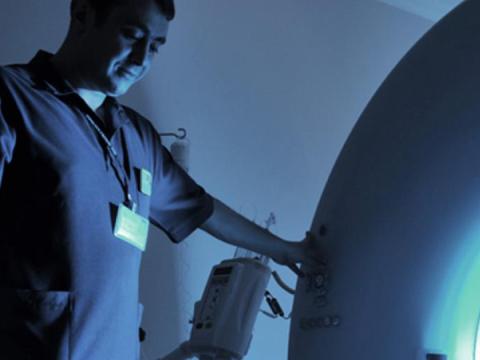
Investigators can work with Baylor College of Medicine’s Office of Clinical Research on Institutional Review Board (IRB) applications, informed consent, and other regulatory requirements for clinical trials. Clinical investigators can also receive assistance with grant budget preparation and negotiation, progress report submission, effort reporting and other post-award administration. OCR provides full clinical trial support and coordination services, including staffing.
Bioethics Support

BCM Center for Medical Ethics and Health Policy | UH Health Law and Policy Institute
Support for Pharmacokinetics, Pharmacodynamics, and Pharmacogenomics (PK/PD/PG)
Support for PK/PD/PG can be found through University of Houston’s Institute for Drug Education and Research or the Gulf Coast Consortia Center for Comprehensive PK/PD & Formulation.
Support for Pharmacokinetics, Pharmacodynamics, and Pharmacogenomics (PK/PD/PG)
Support for PK/PD/PG can be found through University of Houston’s Institute for Drug Education and Research or the Gulf Coast Consortia Center for Comprehensive PK/PD & Formulation.

|
Study Design, Data & Analysis Study design, data and analysis services are offered by Baylor College of Medicine’s Institute for Clinical and Translational Research (ICTR), and the University of Houston’s Texas Institute for Measurement, Evaluation, and Statistics (TIMES) and Hewlett Packard Enterprise Data Science Institute (HPEDSI).
|

|
|
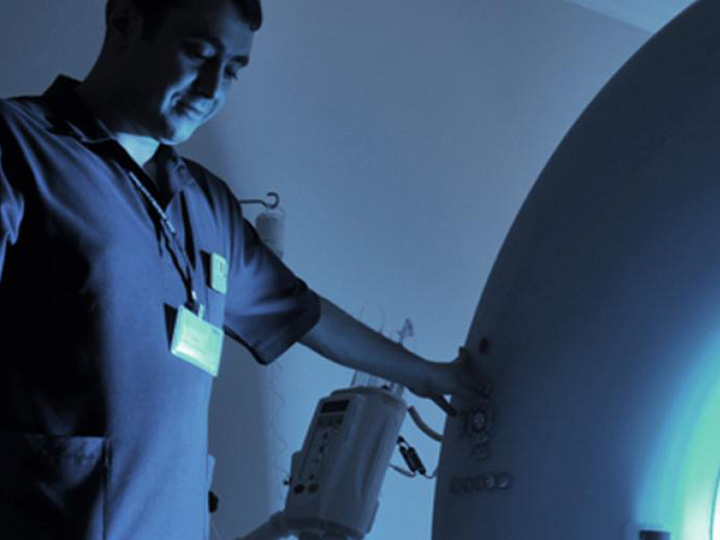
Clinical Trials Support Investigators can work with Baylor College of Medicine’s Office of Clinical Research on Institutional Review Board (IRB) applications, informed consent, and other regulatory requirements for clinical trials. Clinical investigators can also receive assistance with grant budget preparation and negotiation, progress report submission, effort reporting and other post-award administration. OCR provides full clinical trial support and coordination services, including staffing.
|

|

|
Bioethics Support BCM Center for Medical Ethics and Health Policy | UH Health Law and Policy Institute
Support for Pharmacokinetics, Pharmacodynamics, and Pharmacogenomics (PK/PD/PG) Support for PK/PD/PG can be found through University of Houston’s Institute for Drug Education and Research or the Gulf Coast Consortia Center for Comprehensive PK/PD & Formulation. |

|
